Green hydrogen is made when electricity from renewable sources, such as hydroelectricity, wind, or solar power, is used to electrolyze water to produce hydrogen and oxygen. The process, which is called electrolysis, produces hydrogen with no carbon dioxide emissions.
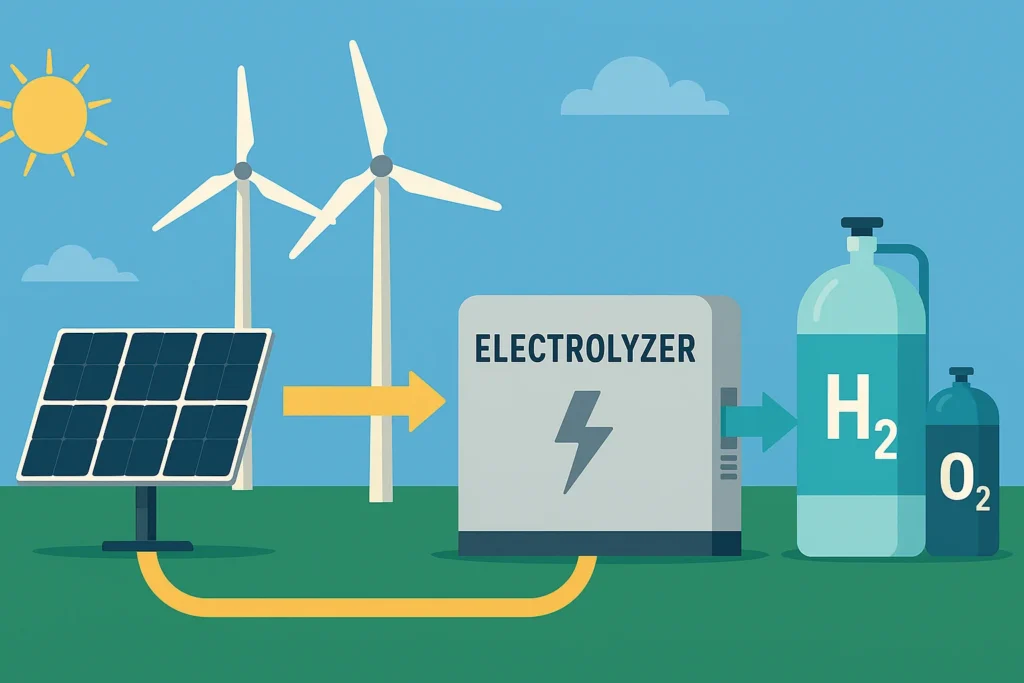
1. Electrolysis Powered by Clean Energy
- Electrolyzers are powered by solar or wind energy and convert water into hydrogen (H₂) and oxygen (O₂).
- If hydrogen is powered by non-renewable resources, it won not be environmentally friendly.
2. Types of Electrolyzers
There are numerous technologies:
- Alkaline Electrolyzers (AEL): Long-lasting and inexpensive; employ liquid alkaline solution (e.g., potassium hydroxide).
- Proton Exchange Membrane (PEM): Small, quick response to power fluctuation; best matched to supporting unpredictable solar or wind, although frequently more expensive because of the employment of noble metal catalysts.
- Solid Oxide Electrolyzers (SOEC): Utilize high temperature for efficiency enhancement but need high-temperature and durable material.
- Anion Exchange Membrane (AEM): Recent technology that attempts to marry the economic benefits of AEL with the flexibility of PEM.
3. Characteristics of the Electrolysis Process
- Water Preparation: Water must be ultrapure to protect the electrolyzer and ensure efficiency.
- Electrolysis Cell: Contains an anode and a cathode separated by an electrolyte—voltage separates the molecules of water.
- Outputs: Hydrogen builds up at the cathode; oxygen at the anode. Hydrogen is stored or utilized.
4.Beyond Electrolysis: New Production Processes
- Photoelectrochemical (PEC) water splitting: Uses specific electrodes and sunlight to directly split water. Currently in progress but is supported by research.
- Biomass/Pyrogasification: Pyro-gasification of waste organic material with limited oxygen supply to produce hydrogen-rich gas—less common but emerging.
5. Why Hydrogen Is Known as “Green”
- Zero-carbon footprint: If electrolyzers are fueled only by renewables, the production of hydrogen emits zero CO₂. By-product? Only oxygen.
- Climate-friendly: Compared to gray hydrogen (fossil-based) or blue hydrogen (carbon capture-based fossil-based), green hydrogen delivers full lifecycle purity.
Frequently Asked Questions (FAQs)
Q1: How much water is consumed for every kilogram of hydrogen?
Ans: It requires about 9 liters of ultrapure water to produce 1 kg of hydrogen.
Q2: What is the most effective type of electrolyzer and why?
Ans: PEM electrolyzers are rapid-response and high-purity and are ideal for integration with renewables. SOECs can become more efficient since heat can be utilized but are high-temperature equipment dependent.
Q3: Is green hydrogen storable?
Ans: In fact — once produced, hydrogen can be compressed or stored in tanks and can also serve as an energy storage medium—sparing excess renewable electricity for later use.
Q4: What are new production technologies of green hydrogen? Ans: Aside from electrolysis, photoelectrochemical water splitting and hydrogen from biomass are still being studied, with potential long-term scalability.
Green hydrogen is at the heart of the energy transition because it offers a zero-emission fuel from water and renewable energy. With existing electrolyzer technologies and new approaches, it’s likely to drive heavy industry, transport, and energy storage.
References
The information in this article is based on insights from respected organizations in the energy field. We have reviewed content from the following sources to ensure accuracy and relevance:
Posted by Abu Talha
With a background in science at the A-level, Abu Talha has studied subjects including physics, chemistry, mathematics, and biology. Along with his more than 1.5 years of experience in digital marketing, he is passionate about writing about electric vehicles, sustainable energy, and how emerging technologies are influencing the future.

